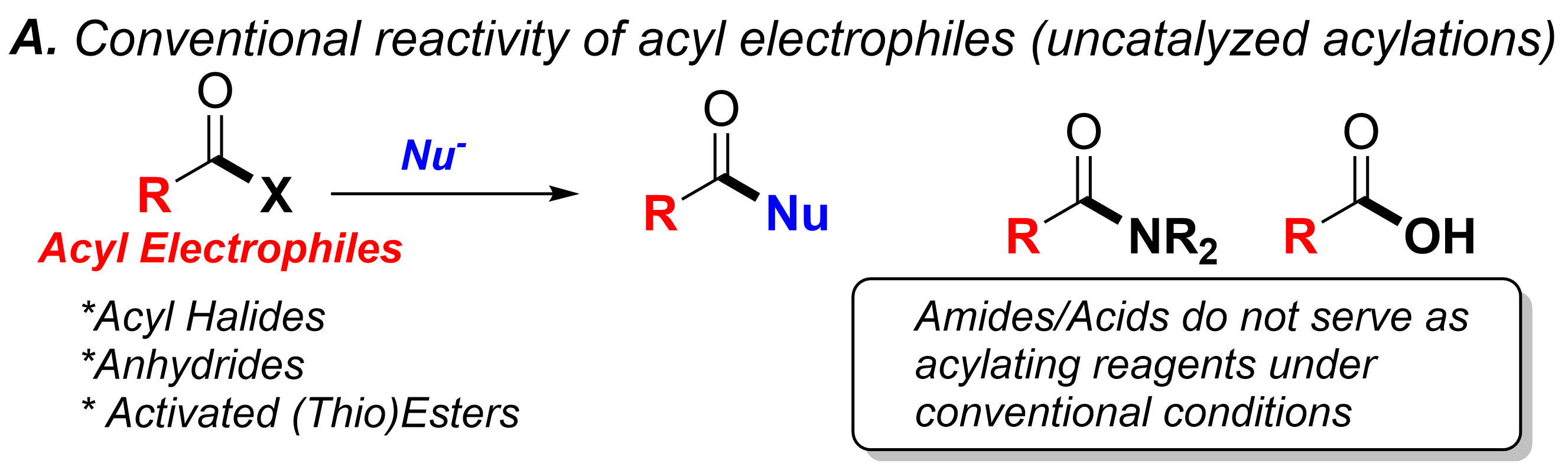
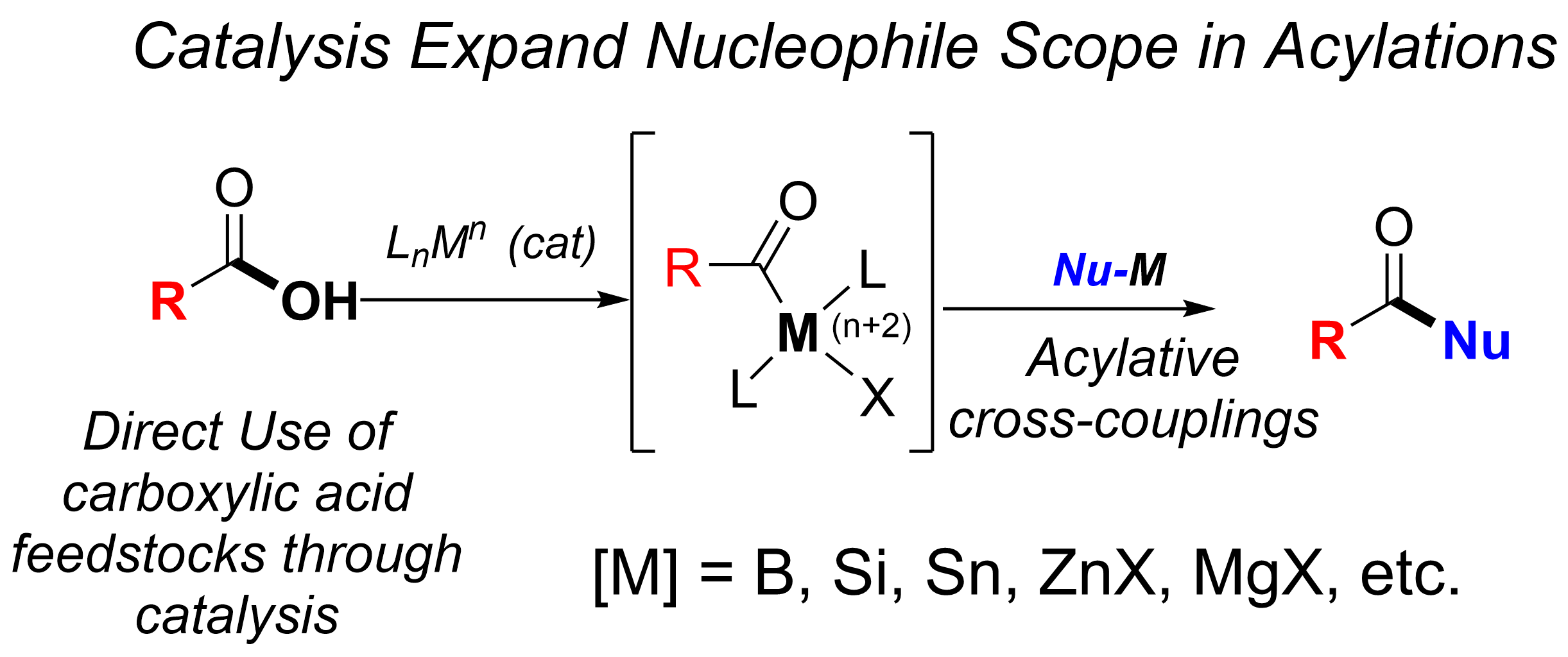
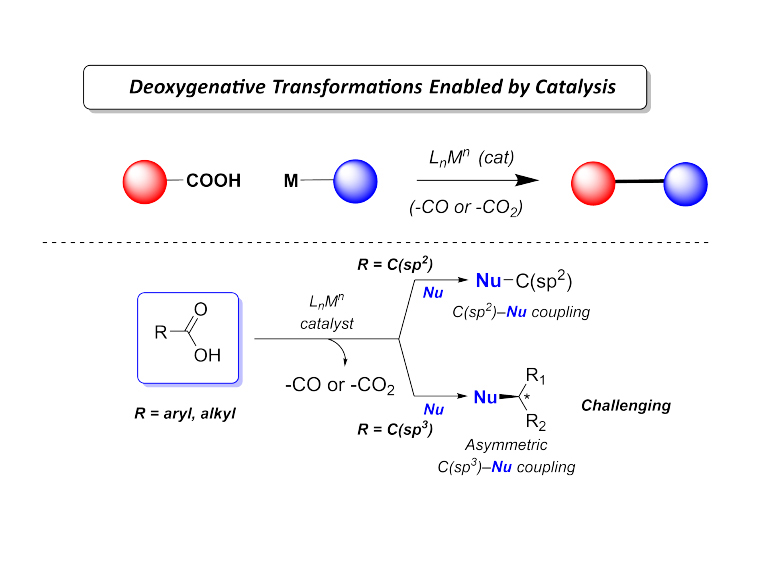
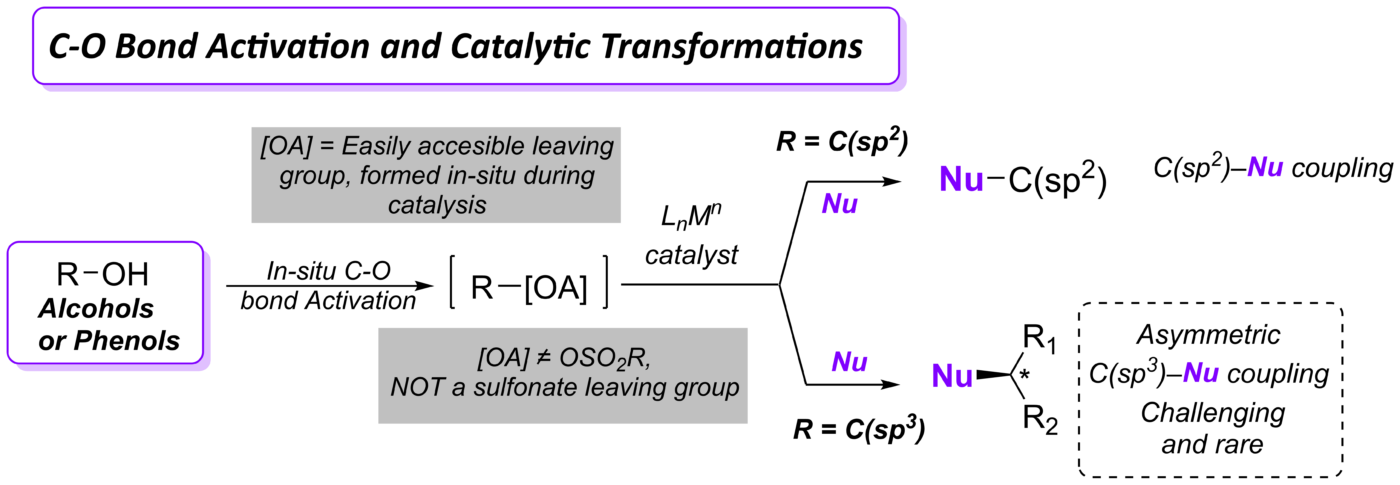
Our group devotes special attention to the synthesis of organofluorine compounds for medicinal applications, organophosphorus chemistry (through P(III/V) mediated/catalyzed processes), SO2 utilization and organosulfur chemistry, carboxylic acid and C–O bond activation protocols. All these areas with a particular aim to the develop catalytic processes.
In addition to organocatalyzed reactions, the PowerCat lab focuses on developing new synthetic transformations by combining the above-mentioned areas with the catalytic power of first-row earth-abundant metals such as Cu, Ni, Co, Fe as well as other valuable transition metal catalysts, either in conventional thermal processes or photoinduced/photoredox processes.

Image: Reaction setup using the new Kessil PRL160L LED lamps. Thanks to @KessilPR for their support.
Our main research areas are:
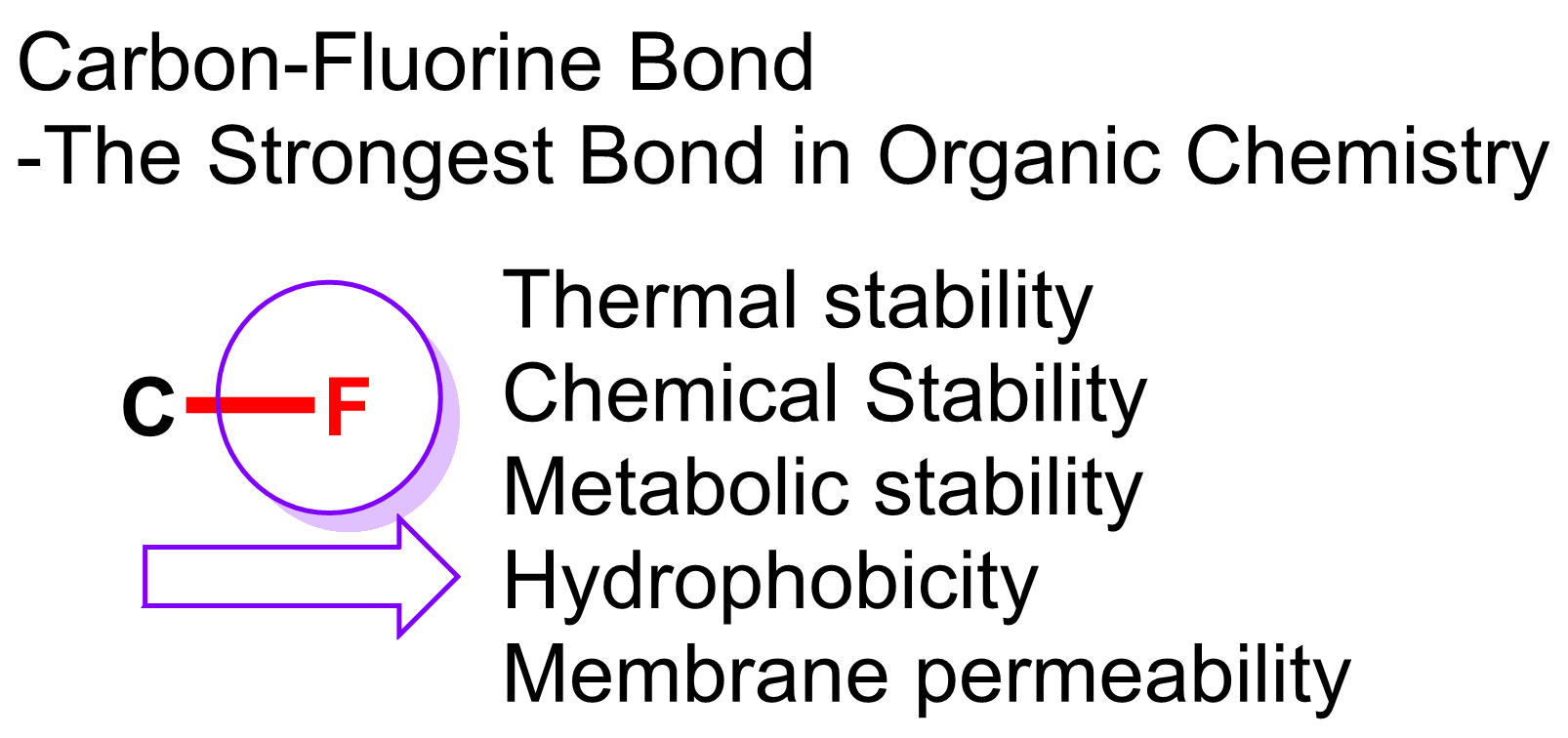
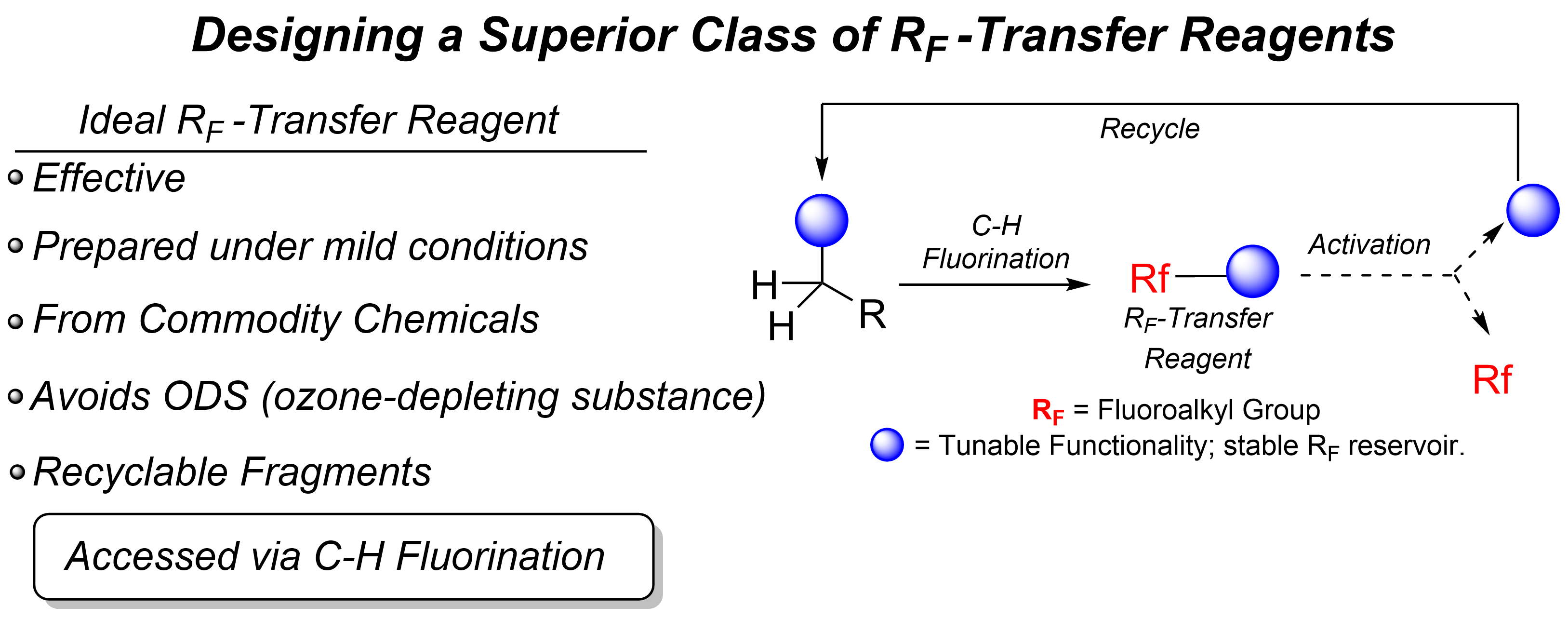
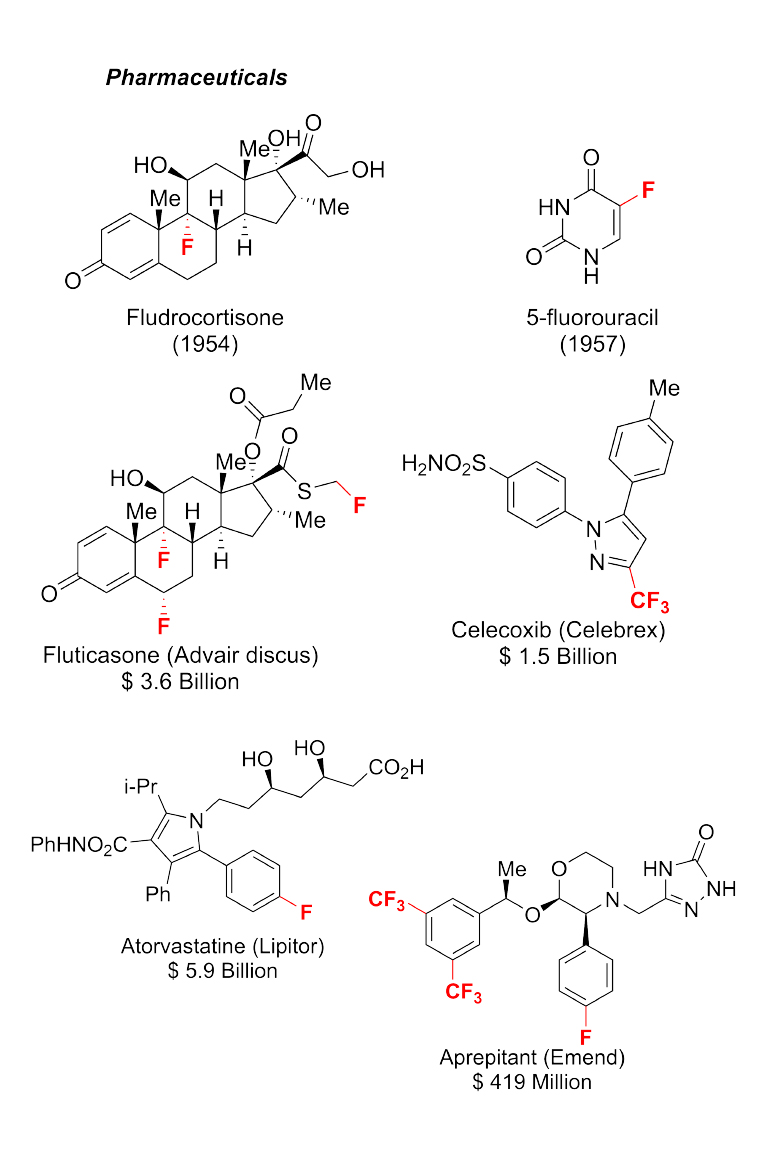
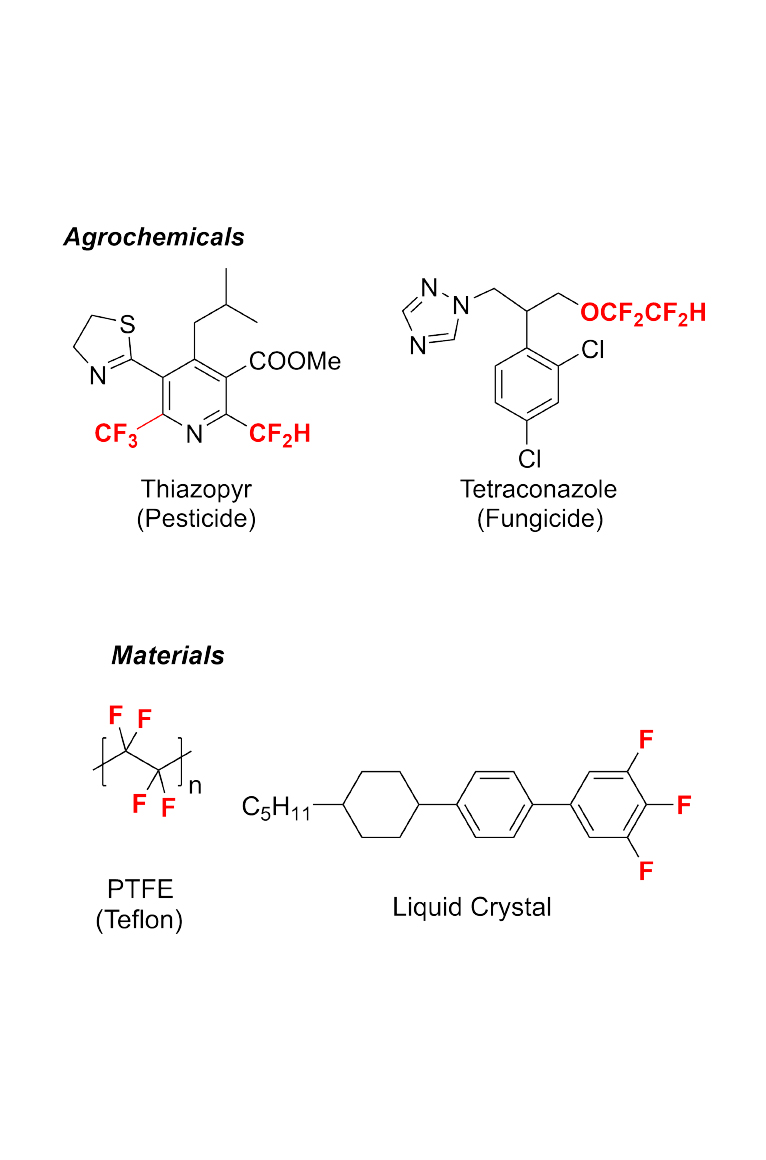
(i) Organofluorine Chemistry: New Reactions and Reagents
(ii) Utilization of carboxylic acids as abundant chemical feedstocks in catalytic synthetic transformations
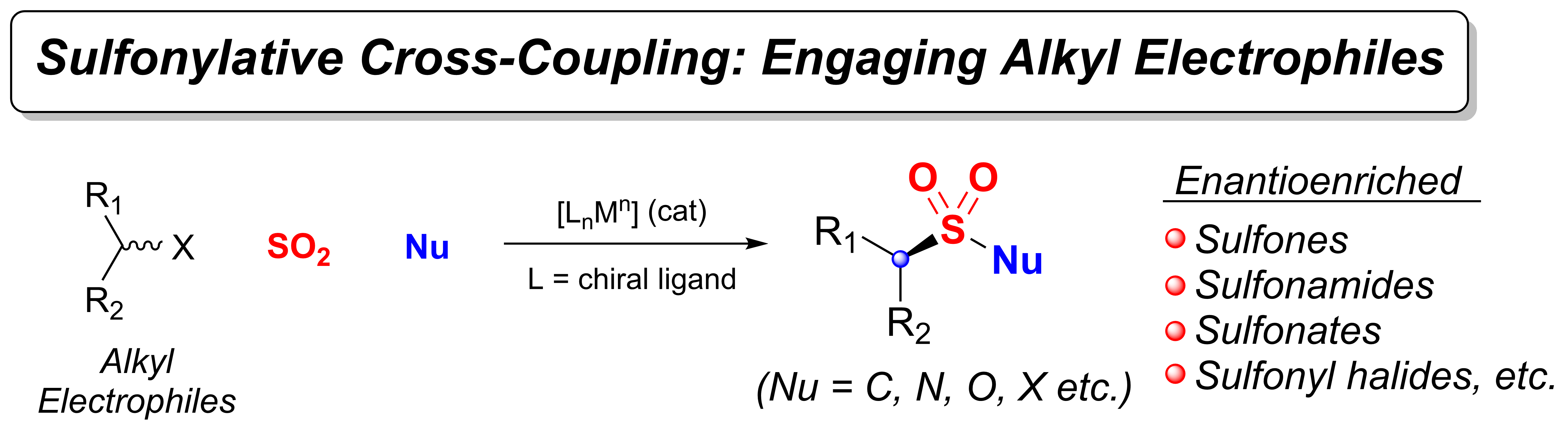
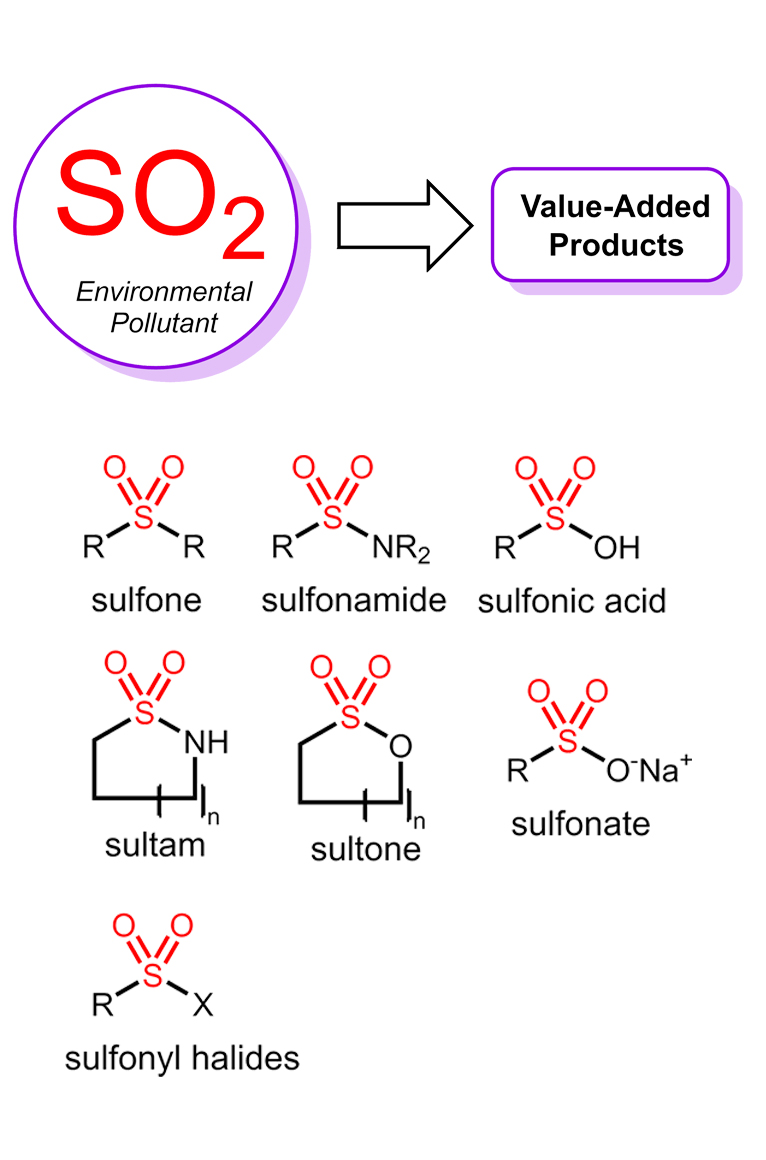
(iii) Utilization of sulfur dioxide (SO2) or chemical surrogates in organometallic catalysis and synthesis.
Development of asymmetric transformations in all the three areas mentioned above stands as a critical unmet need in the field, and special attention is also devoted to this goal. Our group strives to make significant contributions that address the current limitations in those fields. Toward this goal, we continuously utilize of concepts of physical organic chemistry and computations to gain mechanistic insights that facilitate and guide the reaction discovery process. Our ultimate goal is to enable the synthetic community and expand the synthetic chemist’s toolbox. A brief description of the current projects in the group is given below.